Choline: Super Brain Nutrient

Research Roundup:
Nutrition and Traumatic Brain Injury: Improving Acute and Subacute Health Outcomes in Military Personnel.
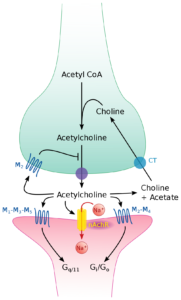
Health outcomes associated with choline involve memory, heart disease, and inflammation, which also explain the consideration of choline as a plausible intervention in traumatic brain injury (TBI)…
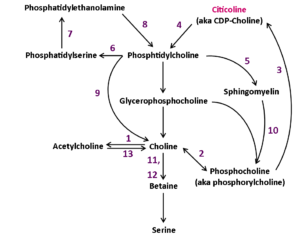
It is hypothesized that CDP-choline may exert neuroprotective effects in an injured brain through its ability to improve phosphatidylcholine synthesis (Adibhatla and Hatcher, 2002). In addition to its neuroprotective capability, CDP-choline potentiates neurorecovery, which has led to its evaluation as treatment for both stroke and TBI in animal models and in human clinical trials (Cohadon et al., 1982; Levin, 1991; Warach et al., 2000). The positive effects seen in models of ischemia and hypoxia may be explained by increased Bcl-2 expression, decreased apoptosis, and reduced expression of pro-caspase. Inhibiting caspase activity may decrease apoptotic activity and calcium-mediated cell death. Supporting these ideas, in vitro studies have also revealed that choline deficiency induces apoptosis in the liver by mechanisms independent of protein 53, which likely involve abnormal mitochondrial membrane phosphatidylcholine, leakage of oxygen radicals, and activation of caspases (Albright and Zeisel, 1997; Albright et al., 1996, 1998, 1999a, 199b, 2003; Chen et al., 2010). In humans, a choline-deficient diet also causes DNA damage and apoptosis (da Costa et al., 2006).
Dosage:
CDP-choline is generally considered safe; the side effect most noted in clinical trials has been mild diarrhea, with leg edema, back pain with headache, tinnitus, insomnia, vision problems, and dizziness reported much less frequently (Adibhatla and Hatcher, 2002; Clark et al., 1997; Levin, 1991). There were no adverse events reported even with doses as high as 4,000 mg/day (Calatayud Maldonado et al., 1991). It is notable that in a study by Clark and colleagues (2001), a dose of 2,000 mg/day by enteral administration did not induce severe adverse events at a rate any higher than placebo in the 899 patients.